Moffett Lab, BTI

|
| Left to right: Saikat Bhattacharjee, Julio Vega-Arreguin, Melanie Sacco, Sarah Collier, Marianne Jaubert, Steven Arias, Peter Moffett. |
Our research is aimed at understanding basic principles governing the function of the plant innate immune system with the long-term goal of applying this knowledge to the generation of disease resistant plants.
Our lab studies the molecular basis of pathogen defense afforded by plant disease resistance (R) genes. Plant R genes are usually dominant and confer resistance to a specific pathogen or pathogen race, including viruses, bacteria, fungi, oomycetes and nematodes. This resistance is dependent on the expression, by the pathogen, of specific avirulence (Avr) proteins that are recognized by the R gene products. A large number of R genes have been cloned and the proteins they encode fall into a small number of protein classes. The most prevalent are the NB-LRR proteins, so named because they possess nucleotide-binding and leucine-rich repeat domains. Of the over fifty characterized R genes encoding NB-LRR proteins, approximately one third have been cloned from Solanaceous plants.
Most Solanaceous R genes will function in other plants of the same family and so we are able to use the model plants Nicotiana tabacum and N. benthamiana, which allow a number of technical advantages. These include Agrobacterium-mediated transient expression for protein function assays and virus induced gene silencing (VIGS) for reverse genetics.
As an experimental model of NB-LRR function, we study the Rx-like proteins of potato. The Rx and Rx2 proteins are highly similar and confer resistance to potato virus X (PVX). The Gpa2 protein is also highly similar to Rx, but confers resistance to the potato pale cyst nematode Globodera pallida. We have undertaken structure-function analyses of these proteins in order to understand the basis of recognition specificity as well as to understand the mechanics of how NB-LRR proteins translate recognition into the initiation of defense responses.
To do so, we are studying the protein-protein interactions that take place within the Rx protein as well between Rx and other cellular proteins. We have recently identified a protein, RanGAP2 that interacts with the Rx-like proteins. RanGAP2 appears to be involved in the recognition process of Rx, Rx2 and Gpa2. We are studying the interaction between the PVX and G. pallida Avr proteins, RanGAP2, and the Rx and Gpa2 proteins in order to understand the molecular basis of recognition and how different recognition specificities have evolved.
Late blight caused by Phytophthora capsici is an increasing problem in pepper production. We have recently started a project whose goal is to apply our knowledge of NB-LRR protein function to the engineering of crop plants resistant to P. capsici by identifying NB-LRR proteins that recognize conserved P. capsici Avr proteins.
Additional projects include the identification of the downstream components required for an effective Rx-mediated anti-viral response and defining how NB-LRR proteins cause viruses to be targeted by this response. To study these questions we use various biochemical, genetic, molecular and cell biology methodologies.
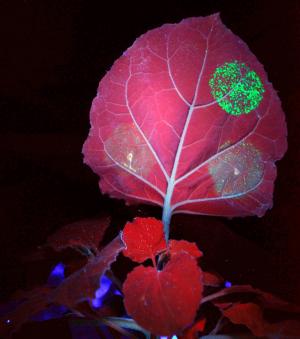
| Nibblet: A virus engineered to express a green fluorescent protein, seen here under ultraviolet illumination, is used to visualize virus spread in the plant. Such tools then allow us to study the plant's ability to recognize, and defend against such pathogens. |

| HR on tobacco: The plant immune system induces cells to undergo a form of programmed cell death upon recognition of pathogen-encoded proteins. In this image, different versions of a pathogen-derived protein have been expressed in patches of a tobacco leaf to study the plant's ability to recognize them. The induction of cell death (brown patch) indicates a successful immune response. |

| Agro-expression: Transient expression of proteins is accomplished by infiltrating an N. benthamiana leaf of with a solution of Agrobacterium tumefaciens carrying a binary vector driving expression from a plant promoter. Proteins of interest are subsequently expressed in all cells of the infiltrated patch and can be assayed for function. In the inset, GFP has been expressed in the infiltrated patches and can be visualized by ultra-violet illumination. |

| Pepper plants uninfected (left), or infected with (right), Phytophthora capsici. |
Contact Details:
Peter Moffett, PhD
Boyce Thompson Institute for Plant Research
Tower Road
Ithaca, NY 14853-1801
phone: 607-254-1362
fax: 607-254-1242
pm99@cornell.edu
http://bti.cornell.edu/PeterMoffett.php
Recent Publications:
Sacco, M.A, S. Mansoor, P. Moffett. 2007. A RanGAP protein physically interacts with the NB-LRR protein Rx, and is required for Rx-mediated viral resistance. Plant J : 82-93
Rairdan, G., P. Moffett. 2007. Brothers in arms? Common and contrasting themes in pathogen perception by plant NB-LRR and animal NACHT-LRR proteins. Microbes Infect : 677-86
Rairdan, G. J., P. Moffett. 2006. Distinct Domains in the ARC Region of the Potato Resistance Protein Rx Mediate LRR Binding and Inhibition of Activation. The Plant Cell 18: 2082-2093
Sawers, R.J., P.R. Farmer, P. Moffett, T. Brutnell. 2006. In planta transient expression as a system for genetic and biochemical analyses of chlorophyll biosynthesis. Plant Methods : 2:15
Moffett, P., J. P. Rathjen. 2003. Early Signal Transduction Events in Specific Plant Disease Resistance. Current Opinion in Plant Biology 6: 300-306
Lu, R., I. Malcuit, P. Moffett, M. T. Ruiz, J. Peart, A. J. Wu, J. P. Rathjen, A. Bendahmane, L. Day, D. C. Baulcombe. 2003. High Throughput Virus-induced Gene Silencing Implicates Heat Shock Protein 90 in Plant Disease Resistance. EMBO Journal 22: 5690-5699
Bendahmane, A., G. Farnham, P. Moffett, D. C. Baulcombe. 2002. Constitutive Gain-of-function Mutants in a Nucleotide Binding Site-Leucine Rich Repeat Protein Encoded at the Rx Locus of Potato. The Plant Journal 32: 195-204
Moffett, P., G. Farnham, J. Peart, D. C. Baulcombe. 2002. Interaction Between Domains of a Plant NBS-LRR Protein in Disease Resistance-related Cell Death. EMBO Journal 21: 4511-4519
Baulcombe, D. C., A. Hamilton, O. Voinnet, R. Lu, J. R. Peart, I. Malcuit, P. Moffett. 2002. Sense and Susceptibility: Dissecting Disease Resistance Using Viruses and Silencing. Biology of Plant-Microbe Interactions 3:
Peart, J. R., R. Lui, A. Sadanandom, I. Malcuit, P. Moffett, D. C. Brice, L. Schauser, D. A. W. Jaggard, S. Xiao, M. J. Coleman, M. Dow, J. D. G. Jones, K. Shirasu, D. C. Baulcombe. 2002. Ubiquitin Ligase-associated Protein SGT1 is Required for Host and Nonhost Disease Resistance in Plants. Proceedings of the National Academy of Sciences, USA 99: 10865-10869